
Efficacy and safety
With Natevba®, precision targeting delivers exceptional results1
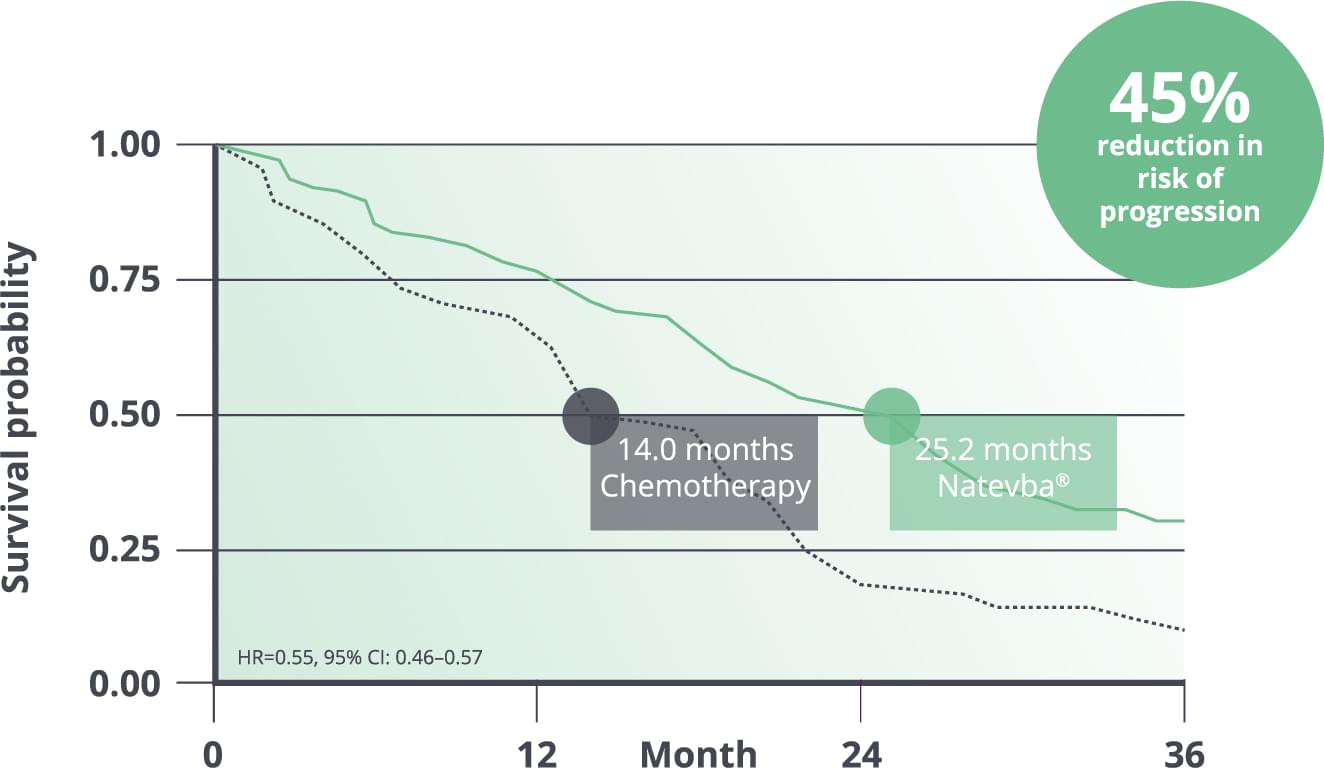
Over 2 years median PFS1

Natevba® delivers over 2 years median PFS.1
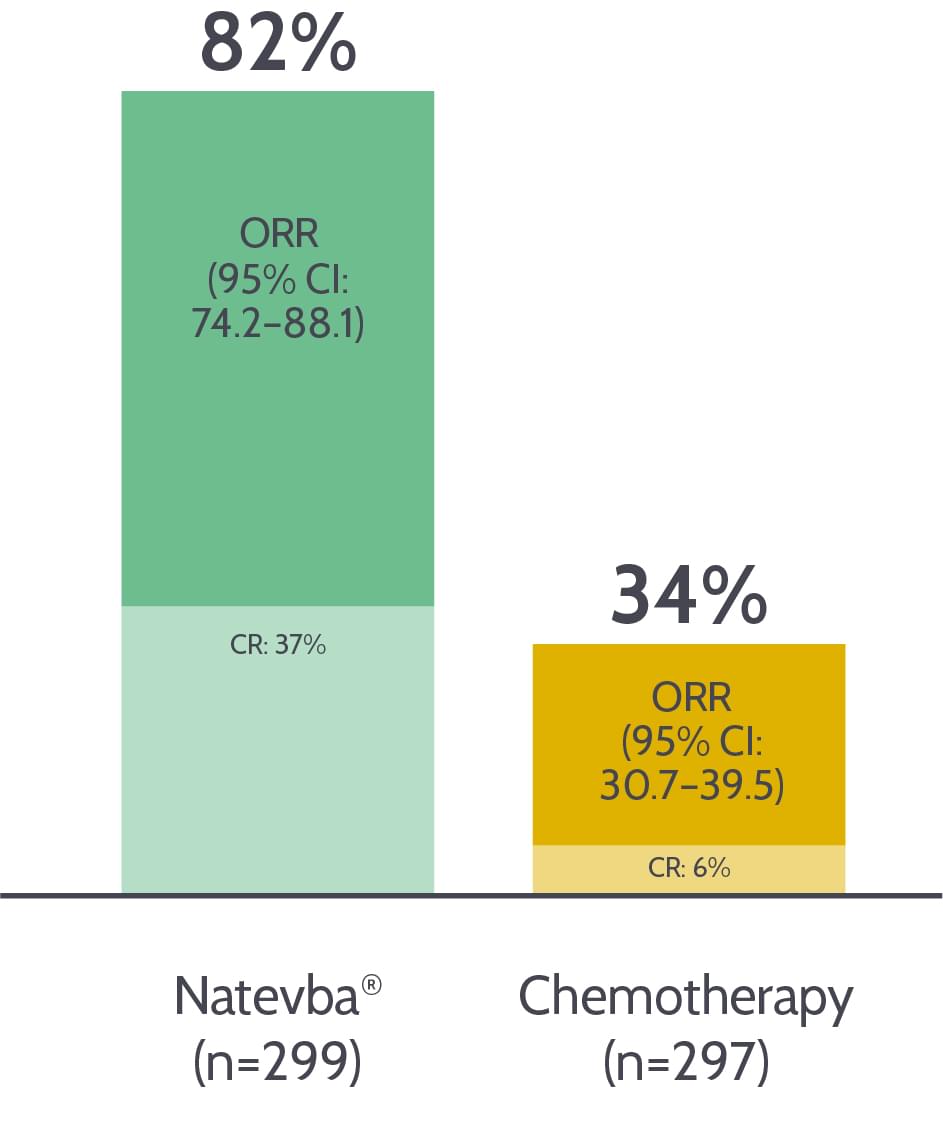
Over 80% response rate1

Over 80% of patients respond to Natevba®.1
Meaningful improvements in QoL1

Natevba® improved or maintained QoL for the duration of treatment in all five EuroQol domains1:
Mobility
Self-care
Usual activities
Pain/discomfort
Anxiety/depression
With Natevba®, precision targeting delivers exceptional results1
Natevba® has a more favourable tolerability profile than chemotherapy, with a lower incidence of Grade 3–4 adverse events1,2
Natevba® has a low incidence of haematological adverse events, including neutropenia1
Adverse events are generally reversible with appropriate dose adjustments1
Natevba®’s manageable dosing schedule lets patients get on with life2
-
Natevba® has a convenient 4-weekly infusion schedule2
- The recommended dose is 1.8 mg/kg administered as an intravenous infusion over 30 minutes every 4 weeks
- Natevba® is indicated as monotherapy, freeing patients from the burden of chemotherapy2
Dose calculator
Patient’s body
weight (kg)
Recommended dose
of Natevba® (mg)